Home / Tech Resource Articles / Product Information / Articles & Downloads /
What is Stainless Steel?
It’s the Chemistry that Makes Stainless Steel “Stainless”
Stainless Steel Chromium Oxide Layer Formation
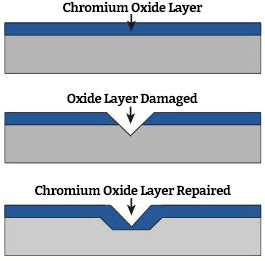
The term “stainless steel” is used to describe more than two hundred different grades, with each one tailored to give outstanding performance in specific applications. All metals react with oxygen in the air or water to form a film or oxide on the surface.
The oxide formed on ordinary steel allows the oxidation to continue producing the typical rusty appearance. However, since stainless steels contain more than 10.5% chromium, the characteristics of the oxide are changed. The chromium reacts with oxygen in the air or water to form the protective passive layer. The passive film is self-healing (in most situations) if damaged. Nickel improves corrosion resistance and formability, and molybdenum improves resistance to localized corrosion.
Stainless steel surfaces can show rusty stains if ferrous contamination on the surface prevents the formation of a continuous passive film. Furthermore, accumulation of dirt may lead to concentrations of corrosive substances that can break down the passive film.